
This pore is associated with events that lead rapidly to necrotic cell death. Under certain types of stress, ANT, VDACs, and other proteins form a nonspecific open channel known as the mitochondrial permeability transition pore. Porins form voltage-dependent anion channels ( VDACs ) through the outer mitochondrial membrane for the diffusion of H 2 O, ATP metabolites, and other ions. ATP synthesized from oxidative phosphorylation is actively transported from the matrix to the intermembrane space by adenine nucleotide translocase ( ANT ). Although oxidative phosphorylation is a mitochondrial process, most ATP utilization occurs outside of the mitochondrion. Proton leakage, chemical uncouplers, and regulated uncoupling proteins increase our metabolic rate and heat generation. In uncoupling, protons return to the matrix by a mechanism that bypasses the ATP synthase pore, and the energy is released as heat. As ATP is used for energy-requiring processes and ADP levels increase, proton influx through the ATP synthase pore generates more ATP, and the electron transport chain responds to restore Δp. The rate of the electron transport chain is coupled to the rate of ATP synthesis by the transmembrane electrochemical gradient. OXPHOS diseases are caused by mutations in nuclear DNA or mtDNA that decrease mitochondrial capacity for oxidative phosphorylation. Mitochondrial DNA ( mtDNA ), which is maternally inherited, encodes some of the subunits of the electron transport chain complexes and ATP synthase. Cytochrome c 1 oxidase, which contains the O 2 -binding site, is inhibited by cyanide. Fatigue can result from iron-deficiency anemia, which decreases Fe for Fe–S centers and cytochromes. Impaired transfer through any complex can have pathological consequences. In cells, complete transfer of electrons from NADH and FAD(2H) through the chain to O 2 is necessary for ATP generation. As protons are driven into the matrix through the pore, they change the conformation of the headpiece, which releases ATP from one site and catalyzes formation of ATP from ADP and inorganic phosphate (P i ) at another site.ĭeficiencies of Electron Transport.
ATP synthase contains a proton pore that spans the inner mitochondrial membrane and a catalytic headpiece that protrudes into the matrix. The pumping of protons generates an electrochemical gradient (Δp) across the membrane composed of the membrane potential and the proton gradient.
#The driving force that pulls protons into the matrix series#
As electrons pass through these complexes in a series of oxidation–reduction reactions, protons are transferred from the mitochondrial matrix to the cytosolic side of the inner mitochondrial membrane. Basically, the electron transport chain contains three large protein complexes ( I, III, and IV ) that span the inner mitochondrial membrane. The chemiosmotic model explains how energy from transport of electrons to O 2 is transformed into the high-energy phosphate bonds of ATP (see Fig. ADP, adenosine diphosphate ATP, adenosine triphosphate NAD, nicotinamide adenine dinucleotide Pi, inorganic phosphate.Ĭhemiosmotic Model of ATP Synthesis. Δp drives protons into the matrix through a pore in ATP synthase, which uses the energy to form ATP from ADP and P i. The positive and negative charges on the membrane denote the membrane potential (Δψ). As electrons pass through the chain, protons are pumped from the mitochondrial matrix to the intermembrane space, thereby establishing an electrochemical potential gradient, Δp, across the inner mitochondrial membrane.
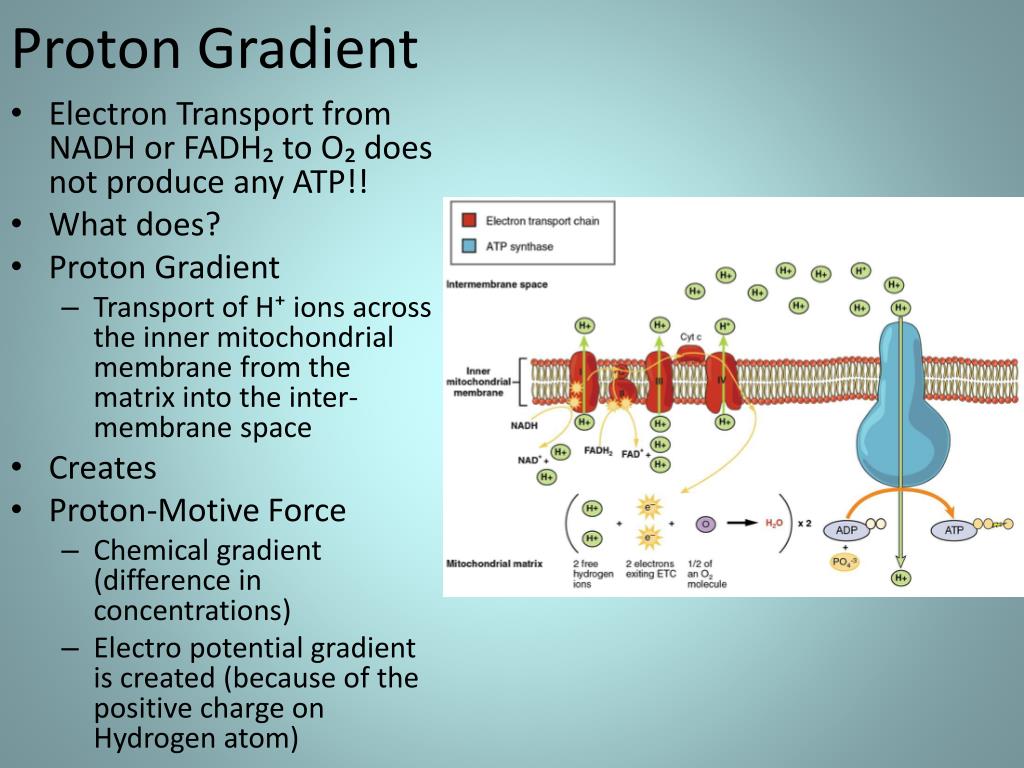
Red arrows show the path of electron transport from NADH to O 2. The net yield of oxidative phosphorylation is ~2.5 mol of ATP per mole of NADH oxidized or 1.5 mol of ATP per mole of FAD(2H) oxidized.įIGURE 24.1 Oxidative phosphorylation. Energy from reduction of O 2 is used for phosphorylation of adenosine diphosphate (ADP) to ATP by ATP synthase ( F 0 F 1 ATPase ). The electron transport chain oxidizes NADH and FAD ( 2H ) and donates the electrons to O 2, which is reduced to H 2 O ( Fig. Most of the energy from oxidation of fuels in the tricarboxylic acid (TCA) cycle and other pathways is conserved in the form of the reduced electron-accepting coenzymes, NADH and FAD(2H).
Oxidative Phosphorylation and Mitochondrial FunctionĮnergy from fuel oxidation is converted to the high-energy phosphate bonds of adenosine triphosphate (ATP) by the process of oxidative phosphorylation.


 0 kommentar(er)
0 kommentar(er)
